Raptar Reporting
Pharmacovigilance Website
Project Description
Project Details
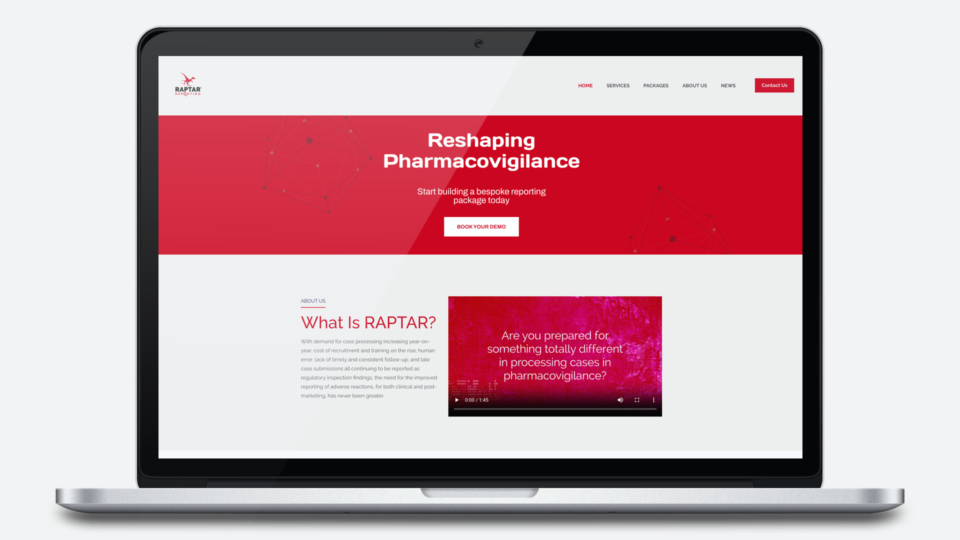
With demand for case processing increasing year-on-year; cost of recruitment and training on the rise; human error; lack of timely and consistent follow-up; and late case submissions all continuing to be reported as regulatory inspection findings, the need for the improved reporting of adverse reactions, for both clinical and post-marketing, has never been greater.
Solutions:
- Responsive Design
- Mobile Ready
- WordPress CMS
- Website Blog Feature
Something in mind? Let’s work on a project
CLIENT
Raptar Reporting
HIGHLIGHTS
Responsive, Web Development